
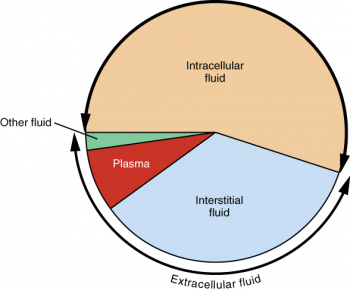
Because these fluids are outside of cells, these fluids are also considered components of the ECF compartment. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Cells are separated from the IF by a selectively permeable cell membrane that helps regulate the passage of materials between the IF and the interior of the cell. Gases, nutrients, and waste materials travel between capillaries and cells through the IF. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Approximately 20 percent of the ECF is found in plasma. The ECF accounts for the other one-third of the body’s water content. An appropriate balance of solutes inside and outside of cells must be maintained to ensure normal function. As a result, water will move into and out of cells and tissues, depending on the relative concentrations of the water and solutes found there. Osmosis is basically the diffusion of water from regions of higher concentration to regions of lower concentration, along an osmotic gradient across a semi-permeable membrane. In the body, water moves through semi-permeable membranes of cells and from one compartment of the body to another by a process called osmosis. For instance, sodium ions (Na +) and chloride ions (Cl –) are often referred to as electrolytes. Often in medicine, a mineral dissociated from a salt that carries an electrical charge (an ion) is called and electrolyte. In the human body, solutes vary in different parts of the body, but may include proteins-including those that transport lipids, carbohydrates, and, very importantly, electrolytes. The dissolved substances in a solution are called solutes. The chemical reactions of life take place in aqueous solutions.
Therefore, to measure the volume of the blood plasma fluid compartment, you need a marker which equilibrates throughout the blood supply and nowhere else. M U is usually calculated from C U, the concentration of marker lost in the urine and V U, the volume of the urine thus: M U = C U. Where V is the volume of the body fluid compartment, M is the mass of marker injected, M U is the mass of marker lost in the urine during equilibration and C is the measured concentration of the marker. As this is not possible (the kidney will excrete everything dissolved in the bloodstream) the calculation must correct for excretion. To be an absolutely perfect marker, the substance should also not be excreted. Therefore: if you know the mass of marker injected into the body and are able to measure the marker concentration once equilibration is complete, you can calculate the volume of the compartment occupied by the marker. Given that concentration (C) = mass (M) / volume (V) it should be obvious that: Furthermore, it must be possible to measure the concentration of the marker once equilibration is complete.Tritiated ( 3H) water is a good marker for the whole body fluid compartment because it diffuses throughout the body, it is chemically identical to normal water and it is easy to measure the equilibrium concentration because 3H water is radioactive.

To be a perfect marker a substance must also not be metabolised. To measure the volume of any fluid compartment within the body you must inject or infuse a marker substance that will equilibrate (diffuse freely to a uniform concentration) throughout this compartment. Body fluid compartments calculations Body Fluid compartments Measuring Body Fluid Compartments


 0 kommentar(er)
0 kommentar(er)
